Research
Carbon dioxide capture and utilization
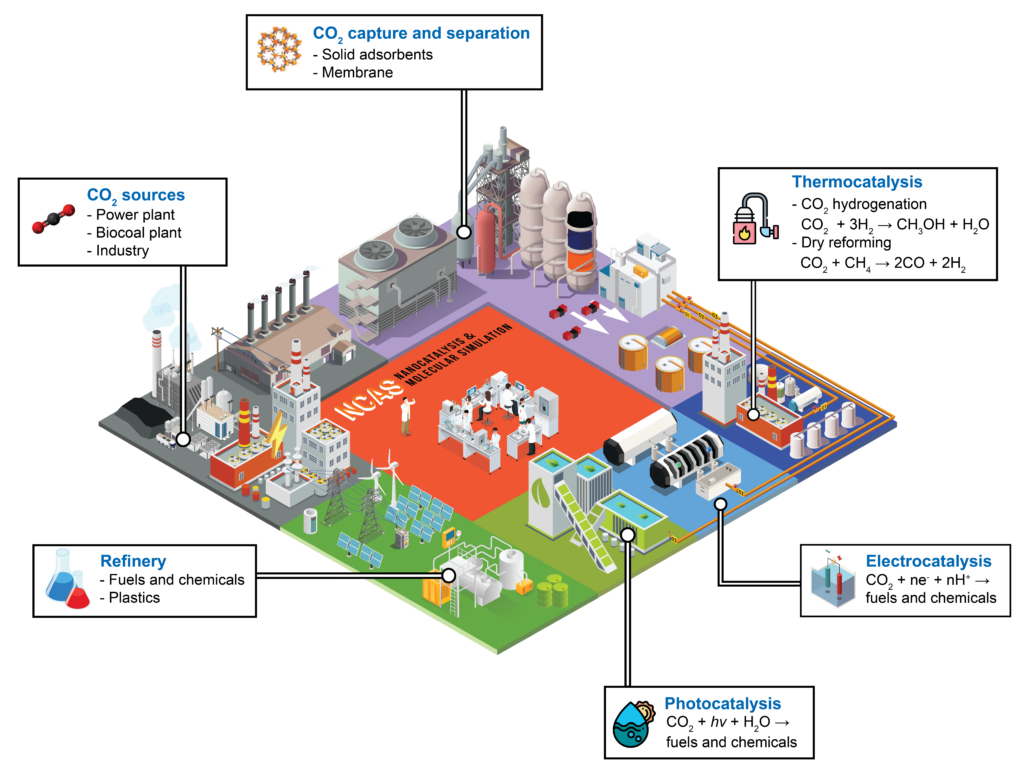
Our research group tackles the entire chain from carbon dioxide capture to utilization. We develop and integrate technologies including solid sorbents, membranes, thermocatalysis, electrocatalysis, and photocatalysis, focusing on both advanced materials and scalable processes. Our goal is to accelerate the transition of these technologies from lab to industry. In parallel, we engage in policy research to inform and support effective frameworks that facilitate the development and adoption CCUS technology in Thailand through Thailand CCUS Alliance (TCCA).
Carbon dioxide capture

Our group develops advanced solid sorbents including metal-organic frameworks, porous carbons, and metal oxides for carbon dioxide capture from point sources as well as other gases and vapors. We work across the full innovation pipeline, from material discovery, performance analysis to scale-up and shaping for practical deployment.
To support this, we are equipped with custom-built reactors and proprietary shaping protocols that enable scalable production of materials with properties tailored to industrial requirements. Our laboratory integrates both commercial and in-house analytical systems, including gas sorption isotherms, breakthrough analysis, and in situ thermogravimetric techniques. These tools allow us to assess sorbents in terms of capacity, thermochemistry, and kinetics with high precision.
Thermochemical CO2 conversion
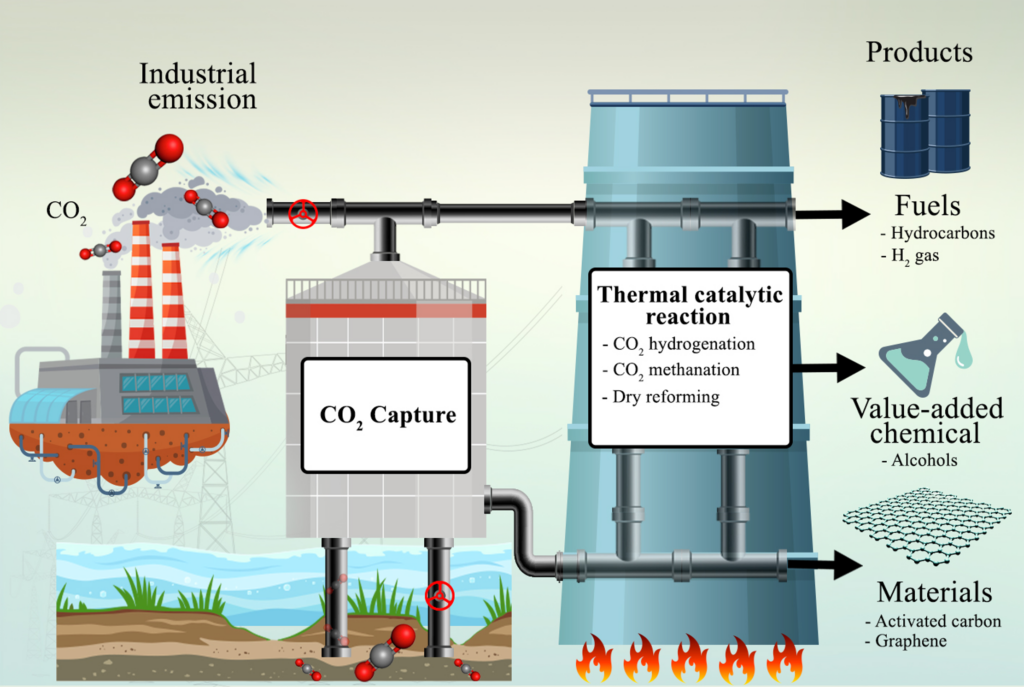
Thermal conversion of CO₂ represents a promising approach for turning a major greenhouse gas into useful chemicals and fuels, contributing to a more sustainable and circular carbon economy. At NCAS, our research explores catalytic pathways such as CO₂ hydrogenation and dry reforming of methane (DRM) to convert CO₂ into value-added products like methanol, syngas, and methane, with a strong focus on designing and optimizing catalysts—including metal oxides and metal-supported nanomaterials—for high activity and selectivity, long-term stability, and resistance to deactivation.
Our laboratory is equipped with comprehensive tools for catalyst synthesis and fabrication, fixed-bed reactor testing, real-time gas analysis, and both in situ and ex situ characterization techniques. By combining material innovation with detailed reaction engineering, we aim to advance thermal CO₂ conversion technologies from the laboratory toward real-world applications.
Electrochemical CO2 conversion

Electrocatalysis technologies driven by renewable electricity are paving the way towards a circular economy. An electrochemical device, consisting of 2 separated half-reactions i.e. oxidation and reduction, offers a unique opportunity for each reaction to be designed and optimized individually. At NCAS, we are designing new electrocatalysts and electrochemical device concepts for chemical, energy, and environmental applications including:
- CO2 reduction reaction (CO2RR)
- Biomass upgrading i.e. oxidation of sugar derivatives
- Pollutant reduction i.e. N2O reduction
- Energy generation and storage i.e. water splitting and fuel cell catalysts
- Disinfectant production i.e. production of H2O2 from O2
Our facility is equipped for material synthesis, electrochemical testing, electrochemical devices and reactors, online product detections, as well as ex situ and in situ characterizations. With a team of experimental and theoretical research scientists, we are taking a combined experimental and theoretical approach to accelerate the development of electrochemical technologies from lab-scale to industrial solutions.
Photocatalysis

Photocatalysis is a process in which light energy is used to drive pairs of chemical reactions. Excited electron (e–) and hole (h+) pairs from light radiation can drive redox reactions such as carbon dioxide reduction, organic waste treatment, biomass fractionation and conversion, and transformation of chemical molecules. Although the concept of photocatalysis is considered as one of the most encouraging technologies, there are some challenging problems related to the photocatalyst materials such as
1) fast recombination rate of e– and h+ pairs
2) wide band gap of semiconductors
3) low stability and reusability of the catalysts.
Our research team is developing novel light absorbers and nanostructured materials which could overcome these limitations for enhanced photocatalytic performance. We focus on low-cost catalysts such as metal oxides, metal sulfides and chalcogenides, metal-organic frameworks, carbon-based materials, and their composites integrated with advanced engineering on catalytic testing systems.


