Meta description (140–160): NANOTEC provides non-animal, standards-based safety testing for cosmetics, cosmeceuticals, and health products. Expert consultation, ISO-aligned methods, and decision-ready reports help you de-risk launches and substantiate claims.
SEO Keywords (one line): cosmetics safety testing, in vitro cytotoxicity, ISO 10993-5, MTT assay, IC50 determination, irritation screening in vitro, non-animal testing Thailand, nanotechnology testing lab, raw material safety evaluation, claims substantiation testing, cell viability assay, cytotoxicity assay
Why This Service Matters
In personal care and health products, safety is non-negotiable. Whether you formulate with botanical extracts, novel actives, or standard cosmetic ingredients, you need evidence that your product is well tolerated and fit for its intended use. Robust in vitro methods give you exactly that without animal testing. Results from validated cell-based assays help you screen ingredients, set safe-use concentrations, compare suppliers, and prepare documentation for internal quality, distributor onboarding, and regulatory review.
At the National Nanotechnology Center (NANOTEC), our Cosmetic & Health Product Safety Testing Services are designed for manufacturers, brand owners, ODM/OEM partners, and R&D labs that want defensible data with clear interpretation. We combine experienced scientists, standards-based protocols, and transparent reporting so you can make decisions quickly and communicate with confidence.
What sets us apart
- Specialist guidance from day one. Our researchers consult on sample preparation, test design, and appropriate cell models so the study reflects real-world use.
- Standards-based, non-animal methods. We use established in vitro techniques and reference internationally recognized frameworks (e.g., ISO 10993-5 for cytotoxicity) where applicable.
- Decision-ready outputs. Data tables, figures, and concise conclusions are packaged for QA systems, customer dossiers, and partner reviews so results can be acted on immediately.
Cytotoxicity & Irritation Screening
Guide price: from THB 24,000 per item (formal quotation confirmed after scoping)
Group 1 addresses toxic and irritation potential using cell-based assays. These methods are suitable for raw materials, botanicals, herbal extracts, cosmetic ingredients, finished cosmetic products, and relevant health-product samples. The goal is to assess whether a test article adversely affects living cells under defined exposure conditions, and when appropriate to estimate concentration thresholds for safe formulation.
1) Cytotoxicity Test (ISO 10993-5 framework; MTT assay; IC50)
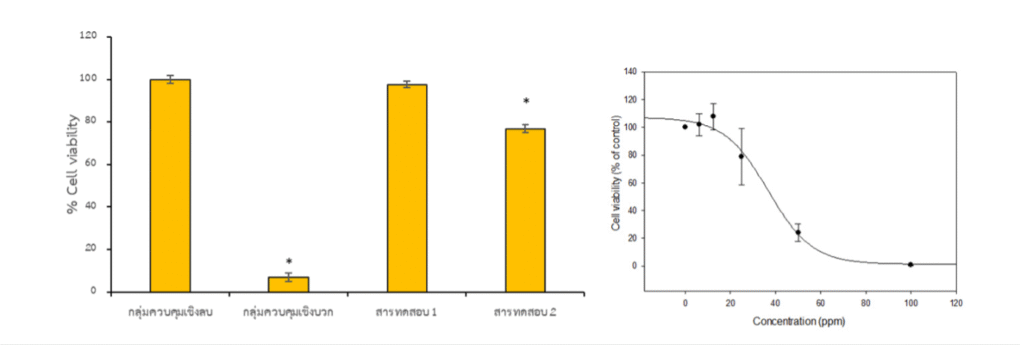
Purpose. To evaluate the effect of a test article on cell viability. Cytotoxicity screening is a reliable first step in characterizing potential irritation or intolerance and is often used to guide ingredient levels during formulation.
Principle. Cells are cultured and exposed to the test article across a dose-response series. Cell metabolic activity (a proxy for viability) is quantified by MTT assay, in which viable cells reduce MTT to a colored formazan product. Absorbance is measured and normalized to controls to calculate % cell viability.
Standards reference. The study can be designed with reference to ISO 10993-5 (Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity).
Dose design. At least five concentrations are tested to generate a response curve suitable for calculation of IC50 (the inhibitory concentration that reduces viability to 50%).
Controls. Appropriate negative and positive controls are included to ensure the assay performs as expected and to contextualize results.
Outputs.
- % cell viability at each tested concentration
- IC50 value (when calculable from the curve)
- Observation notes (e.g., precipitation, color interference)
- Interpretive statement indicating whether the sample shows cytotoxicity under the specified conditions
Suitable sample types.
- Raw materials: oils, waxes, surfactants, emulsifiers, polymers
- Botanicals & extracts: solvent or CO₂ extractions, tinctures, standardized actives
- Finished products: creams, serums, gels, cleansers, toners
- Health-product formats with topical or cosmetic-adjacent use
Use cases.
- Screening new or reformulated ingredients before scale-up
- Establishing maximum use levels with a safety margin
- Supporting “non-irritating” positioning alongside other evidence
- Comparing supplier lots or extraction conditions
- Rapidly triaging out-of-spec or complaint investigations
Note. If a sample is highly colored, opaque, or reactive in ways that may interfere with optical readouts, we will flag it during scoping and discuss mitigation (e.g., dilution windows, blank correction).
How a Study Is Planned & Executed
1) Scoping & Method Fit
We start with a short scoping discussion to align on the intended use, sample composition, and exposure scenario (e.g., rinse-off vs. leave-on; neat vs. diluted). From there, we confirm:
- Test article handling (e.g., direct application, solvent dilution, extraction medium)
- Concentration range and spacing across ≥ 5 points
- Exposure duration appropriate for the product type
- Control scheme and replication plan
This ensures the data generated are both scientifically sound and business-relevant.
2) Sample Receipt & Preparation
- Samples are logged, inspected, and stored under conditions you specify.
- If dilution or extraction is required, we will prepare test solutions accordingly and document the parameters.
- For solid or viscous products, specific handling (e.g., homogenization) will be agreed during scoping.
3) Cell Exposure & MTT Readout
- Cells are seeded and allowed to adhere and equilibrate.
- Test solutions are applied for the planned exposure period.
- After treatment, MTT reagent is added; viable cells convert it to formazan.
- Formazan is solubilized and the absorbance measured; results are normalized to controls to yield % viability.
4) Data Reduction & IC50
- Dose-response curves are fit with appropriate models.
- IC50 is reported (when curve behavior permits) and accompanied by goodness-of-fit diagnostics.
- Outlier checks and control acceptance criteria are applied according to the method.
5) Review & Reporting
We compile a clear, decision-ready report that contains the information your QA and product teams need.
What You Receive: Reporting You Can Use
Every project concludes with a concise, structured report:
- Executive summary. One-page overview of objectives, methods, and key conclusions.
- Results table. % cell viability at each concentration; IC50 where applicable.
- Dose-response figure. Plot with confidence shading for fast visual interpretation.
- Controls and acceptance. Negative/positive control results and pass/fail statements.
- Method summary. Reference to ISO 10993-5 framework; assay conditions (cell model, exposure time, readout).
- Sample details. Identification, batch/lot (if provided), and any special handling.
- Interpretive notes. Practical guidance such as safe initial use ranges to explore, or whether further testing is advisable.
Reports are formatted so they can be shared with partners or filed in internal systems without rework. Editable data files can be provided on request.
Sample Guidance to Ensure Valid, Reproducible Data
Good results start with good samples. Please follow these tips:
- Send representative material in the form closest to use (raw, extract, or finished product).
- Avoid contamination (e.g., silicone oil, fingerprints). Use clean, inert containers with tight closures.
- Provide solubility context (e.g., water-soluble, ethanol-soluble, oil-soluble), and list any hazards so we can handle safely.
- Specify storage requirements (light sensitive, refrigerated, etc.).
- Quantity. Provide enough for the full concentration series and repeats; we will confirm required amounts during scoping.
- Label clearly. Include sample name, lot number, and concentration (for solutions).
- Share your goal. Tell us if you are screening, comparing suppliers, or preparing claim support this influences dose choices and how we present results.
Typical Applications by Product Stage
- Early R&D. Screen multiple botanical extracts or synthetic actives to focus development on ingredients with acceptable cytotoxicity profiles.
- Formulation optimization. Compare base formulas with and without a suspect component to identify drivers of irritation potential.
- Supplier qualification. Confirm that incoming raw materials from a new vendor perform similarly to your reference lot.
- Change control. Document that a process change (e.g., extraction solvent, neutralizer selection) does not materially affect cell tolerance.
- Complaint resolution. Use cytotoxicity data as part of a multifactor investigation when field reports indicate redness, tightness, or stinging.
Who This Service Is For
- Cosmetics & Cosmeceutical Brands. Generate dossiers for internal review and trade partners; substantiate “gentle,” “non-irritating,” or “sensitive-skin suitable” positioning when used alongside clinical evidence.
- ODM/OEM Manufacturers. Offer customers a more complete technical package and shorten decision cycles during formulation sign-off.
- Ingredient Suppliers. Provide customers with concentration guidance and safety screens to differentiate your botanicals or actives.
- SMEs & Startups. Get affordable, non-animal safety data to de-risk scale-up and retail onboarding.
- Universities & Research Institutes. Obtain standardized cytotoxicity data to support publications or translational projects.
Pricing & Quotation
- Guide price: from THB 24,000 per item for Group 1 cytotoxicity/irritation screening using the MTT assay framework.
- The final quotation depends on sample count, number of concentrations, replication, and any special handling (e.g., solubility constraints).
- Multi-sample programs and comparative studies (e.g., A/B supplier lots) can be consolidated into a single report to streamline internal review, please ask during scoping.
Quality Framework
- Studies are run under documented laboratory procedures with traceable parameters and control acceptance criteria.
- Methods align with non-animal testing principles and reference ISO 10993-5 for cytotoxicity.
- We maintain transparent communication throughout the project if we observe potential assay interference or unexpected behavior, you will be notified and options discussed.
Frequently Asked Questions
What exactly does the cytotoxicity test tell me?
It quantifies how your sample affects the viability of cultured cells under defined exposure conditions. The outputs % viability and IC50 are used to compare ingredients, evaluate dose ranges, and flag potential irritation concern.
Is the method suitable for complex finished products?
Yes. We will agree on dilution or extraction steps appropriate to your product type and intended use so that results are meaningful.
Do you use animals?
No. The approach is in vitro (cell-based) and does not involve animal testing.
Can I combine this with other efficacy tests?
Yes. Many customers run safety screening in parallel with efficacy-oriented assays. Tell us your plan and we will help you sequence the work efficiently.
How much sample do I need to send?
This depends on the concentration series and repeats; we will confirm during scoping. As a rule of thumb, plan for enough material to prepare ≥ 5 concentrations with triplicate wells, plus controls.
Can I share the report with partners and distributors?
Absolutely. Reports are written in plain, decision-focused language with data tables and figures suitable for partner packages.
How to Get Started
- Share your objective. A brief note about product type, ingredients of interest, and intended claims helps us recommend a fit-for-purpose design.
- Confirm scope & quote. We’ll outline the test plan (concentrations, exposure time, replication) and issue a formal quotation.
- Ship samples. Follow the Sample Guidance above to avoid delays.
- Receive results. You’ll get a clear report with % viability, IC50, and concise interpretation tied to your goal.
NANOTEC’s Cosmetic & Health Product Safety Testing Services give you the non-animal, standards-based evidence you need to move products forward with confidence. From raw materials and botanicals to finished formulas, our cytotoxicity screening built on ISO 10993-5 methodology and MTT readout provides quantitative, defensible data such as % viability and IC50. With expert consultation, careful method design, and reports written for decisions, you can de-risk development, substantiate claims, and communicate clearly with customers, distributors, and regulators.


