National Science and Technology Development Agency | National Nanotechology Center
Research
Energy storage
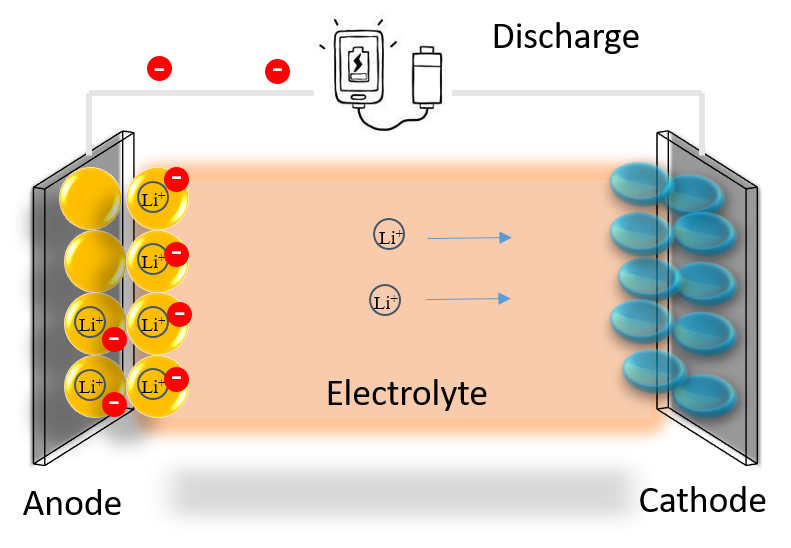
Would you be interested in cell phones, laptops, and other electronic devices that can last for a couple days without dying? Or what about electric cars with long range that can do road trips of several days without needing a battery recharge? If you answered “yes” to either of these questions, then you may be interested in our research, which focuses on finding ways to improve the efficiency of batteries, a crucial step towards achieving these applications. Lithium-ion batteries are a type of secondary (rechargeable) battery that can be discharged to store lithium ions in the anode (negative electrode) of a battery cell like in the figure shown below. When you use the battery, Li ions will run from the anode to the other side which is called the cathode (positive electrode), releasing electrons that power your electronic devices. The electrons and Li ions will then combine and are stored together in the cathode. If you can store more Li ions in the electrodes, more electrons can be released, providing more juice for your devices: we call this term high energy density.
One way to achieve higher energy density is to develop new materials that can store a lot of Li ions. Our works focuses on utilizing agricultural and industrial waste to develop electrode materials. We use materials such as activated carbon, silicon composites, heteroatom doped materials, and nanostructure materials within Li-ion and Li –sulfur batteries to achieve higher performance than regular batteries. Beyond the electrodes, other parts of batteries also need to be improved to make future batteries, and therefore we are also interested in making new materials for polymer binders, separators, and electrolytes. If you want to help make the world a better place through clean energy and are interested in discovering new battery inventions, please contact us for more information!
